Next-Generation Artificial Pancreas System Launches
Medtronic’s New MiniMed®640G Artificial Pancreas System Predicts and Helps Prevent Deadly Low Glucose Emergencies
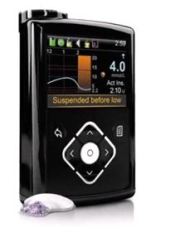
A four year-old Australian boy with type 1 diabetes (T1D) is the first person to use a groundbreaking commercial artificial pancreas system that can predict and automatically prevent low blood-glucose emergencies. Medtronic’s MiniMed® 640G is an insulin pump/continuous glucose monitor combination that uses newly developed software to predict the user’s future glucose levels. If the user doesn’t respond to alarms indicating that levels are trending too low, the MiniMed® 640G automatically shuts off insulin delivery to minimize the chances of blood-glucose levels falling to dangerously low levels, a condition called hypoglycemia. Once the person’s glucose levels stabilize and at least 30 minutes have passed since the last insulin dose, the system resumes insulin delivery to prevent a damaging high blood-glucose level rebound.
“Having this new system gives us the reassurance that Xavier is safe when we are all asleep at night, and during the day” said Naomi Hames, mother of MiniMed 640G recipient Xavier Hames. “It is also waterproof, meaning that he can enjoy water sports and activities as much as his friends and family.”
The MiniMed® 640G is classified as a predictive low glucose suspend artificial pancreas system, the second step in the JDRF artificial pancreas strategic development plan. It’s an improvement over currently available low glucose suspend systems, which suspend insulin delivery only when a person’s glucose levels drop below pre-set thresholds. Currently available first generation artificial pancreas systems can’t predict future hypoglycemic emergencies.
Predictive Low Glucose Suspend technology is especially effective in reducing the chances of potentially deadly overnight hypoglycemic emergencies. Studies have found that more than half of all hypoglycemic emergencies occur overnight, with sleeping users (and their loved ones) failing to respond to 70 percent of their glucose sensor’s warning alarms. While the technology does not address blood glucose highs, it is a further step toward the ultimate goal of a fully automated artificial pancreas system.
The Medtronic MiniMed® 640G is currently available only in Australia. Medtronic expects further launches of the system around the world over the next 12 – 18 months.
For more information or to support JDRF’s artificial pancreas research program, please click here.